
Guide for Household/Urban Hazardous Substances Registration in the Philippines
The demand for innovative household substances has significantly risen in the Philippines through the years. From disinfectants and multi-purpose cleaners to home and car fresheners and aerosol sprays, consumers now look for effectiveness, product safety, cleanliness, and clear FDA registration status. As social media evolves, the masses are easily influenced by recommended products, trends, and “must-have” items promoted for daily consumption.
However, as the products are more accessible in public, there may be a potential risk to health since some products contain corrosive, toxic, and flammable substances. To safeguard the public, Household/Urban Hazardous substances must comply with the regulations set by the Food and Drug Administration to ensure that the products are safe, assessed and approved before it can be sold to the Philippine market.
- What are HUHS Products?
- What are Examples of Household/Urban Hazardous Substances?
- What is the Content of Product Labeling and Packaging for HUHS?
- Steps for Household/Hazardous Substances Registration in the Philippines
- Common Deficiencies and Reason for Application
- Validity and Renewal of License to Operate and Certificate of Product Registration (CPR)
- Frequently Asked Questions
What are HUHs Products?
As per FDA definition “Household/Urban Hazardous Substance (HUHS) refers to any substance or mixture of substances intended for individual or limited purposes and which is toxic, corrosive, an irritant, a strong sensitizer, is flammable or combustible, or generates pressure through decomposition, heat or other means, if such substance or mixture of substances may cause substantial injury or substantial illness during or as a proximate result of any customary or reasonably foreseeable ingestion by children, but shall not include agricultural fertilizers, agricultural pesticides, and agricultural insecticides, and other economic poisons, radioactive substance, or substances intended for use as fuels, coolants, refrigerants.
What are Examples of Household/Urban Hazardous Substances?
List of Category III HUHS Product (Cleaners, Fresheners and Deodorizers)
- Bleaches
- Cleaners
- Deodorizers
- Dishwashing and Laundry Detergents/Soaps
- Disinfectants (for surfaces)
- Fabric Conditioners/Softeners and Ironing Aids
- Fresheners (i.e. room, car, etc), Aromatics, Diffusers
- Moisture Absorbing Agents (i.e. desiccant)
- Polishes
- Pool Chemicals
List of Category IV HUHS Products (Do-It-Yourself and Hobby Items)
- Adhesives, Glues and Sealants
- Automotive, Furniture and Jewelry Care, and Restoring Products
- Button Batteries
- Coloring Materials
- Fabric Dyes, Tattoo Dyes
- Paints, Varnishes, and Thinners
- Paint Stripper
- Rust Remover/Degreasers
What is the Content of Product Labeling and Packaging for HUHS?
- Product Information – Ensure that the HUHS product must bear a label such as brand and product name, full ingredient list, batch number, manufacturing and expiry date.
- Direction for Use – Indicate the product direction of use as reflected in the Label.
- Handling, Storage and Disposal – Indicate the handling, storage and disposal instructions applicable to the product.
- Company’s complete name, Address and Contact Information of Marketing Authorization Holder – The name, address and contact information should be consistent with the declared distributor in the application form.
- Country of Manufacture – Should be consistent with the source of information in the application form and technical requirements.
- Net Content – Indicate the quantity of the product contained in the packaging. This must be expressed in metric units (e.g., mL, L, g, kg) and should be clearly visible on the principal display panel.
- Hazard Information – Indicate the nature of the hazard based on the product’s GHS classification.
- Product Category – Specify whether the product is Ready-to-Use or For Professional Use.
- Signs / Symptoms of Poisoning – List the signs and symptoms that may occur due to accidental or undue exposure to the product (e.g., skin irritation, nausea, difficulty breathing).
- First-Aid Treatment / Medical Advice – Provide clear instructions for initial care in case of exposure (e.g., rinse with water, and seek medical attention).
- Contact Information of the National/Regional Poison Center – Include the contact details of the National Poison Management and Control Center and the relevant regional poison centers.
Steps for Household/Hazardous Substances Registration in the Philippines
- Secure License to Operate
International or Local companies who plan to manufacture, distribute, wholesale and trade Household/Urban Hazardous Substances are required to obtain License to Operate to ensure that the establishment is compliant with the FDA regarding safety handling of HUHS products
- Identify the Product Category
Identify whether the product will fall into Categories III and IV HUHS products and should be further classified as either:
(a) Ready-to-Use – products that are for general purposes; the products require no further dilution prior to application and are not restricted to trained personnel or professionals.
(b) For Professional Use – products that are highly concentrated require further dilution and should only be applied by trained personnel.
- Secure Certificate of Product Registration
Once the LTO has been successfully secured, the application of the CPR may proceed following the requirements:
-
- License to Operate
- Certificate of Analysis
- Product Label and Artwork
- Safety Data Sheet
- Proforma Invoice or Distributorship Agreement
- Product Formulation
- Post-Approval
Prepare all the Certificates and requirements for inspection by the FDA. Ensure that the establishment is complying with Good Distribution Practice.
Common Deficiencies and Reason for Application
The application may result in denial due to the following reason:
- Incomplete or Missing Technical Documentation
Missing or attaching incorrect document required attachment for the registration
- Product Claims and Labelling Deficiency
- Product claims that are lacking substantiation and not supported by and scientific evidence
- Product labels that do not comply follow GHS Labelling guidelines, including missing pictograms, signal words and hazard statements
- Ingredient and Formulation Limit
Product ingredient that exceeds the maximum concentration and allowable limit.
- Product Safety & Non-Compliance to Regulations
Product that doesn’t have risk assessment data and no safety documentation.
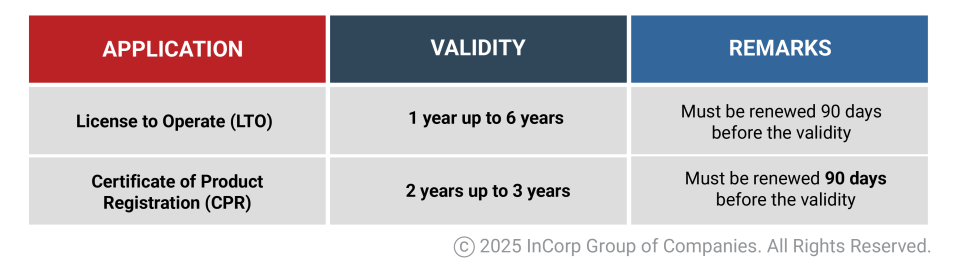
Validity and Renewal of License to Operate and Certificate of Product Registration (CPR)
Let Us Help Register Your Household/Urban Hazardous Substances with the FDA
Frequently Asked Questions
Which center of FDA does HUHS products fall under?
The Food and Drug Administration (FDA) regulates HUHS products under the Center for Cosmetics and Household/Urban Hazardous Substances Regulation and Research (CCHUHSRR).
Is product registration required before selling HUHS in the Philippines?
Yes, HUHS products are required to be registered and shall have a valid Certificate of Product Registration (CPR) before they can be legally sold, imported, exported, distributed in the Philippines.
Can I import HUHS products without FDA registration?
No. Importation of HUHS products for commercial sale in the Philippines requires that the importer holds a valid LTO and CPR.
Is a Safety Data Sheet (SDS) always required?
Yes, a Safety Data Sheet (SDS) is always required for HUHS product registration, and it must follow the GHS format with complete information applicable to all product variants.
Are GHS pictograms required even for imported products?
Yes, GHS pictograms are required even for imported HUHS products, and labels must comply with FDA standards
What if a HUHS product is marketed in different sizes or packaging?
HUHS products must be registered on a per formulation basis. HUHS products intended to be marketed in multiple pack sizes and/or packaging materials may be registered together in a single initial HUHS product registration application.
Can I use a single SDS for multiple variants of a HUHS product?
Yes, provided that all variants are clearly identified in the SDS and the information applies to all of them.
Can I use the term “non-toxic” or “safe” on my HUHS product label?
No. Labels must not bear unsubstantiated safety claims such as “safe,” “non-toxic,” “non-hazardous,” or similar terms.
What happens if I want to change the product’s formulation after approval?
Any change in product formulation or manufacturing site requires a new initial HUHS product registration application.
Can I include the FDA logo or the words “FDA approved” on my product label or marketing materials?
No. Use of the FDA logo or any imitation of it is strictly prohibited.



Hello, Could you please advice if HUHS registration is required for Commercial and Industrial use only cleaning chemicals that are not intended for household use?
HUHS registration is not required for cleaning chemicals intended for industrial use. FDA registration applies only to HUHS products for consumer or institutional use.