
Guide For Medicine/Drug Registration in the Philippines
As healthcare continues to evolve, ensuring medical products are safe and effective is essential. In the Philippines, the Food and Drug Administration (FDA) oversees the legal requirements of drug registration to ensure that all pharmaceutical products meet the standards for quality, safety, and efficacy. Before any pharmaceutical product can be sold legally in the Philippines, a Certificate of Product Registration (CPR) must be obtained, regardless of whether you are a domestic manufacturer, importer, or distributor.
- What is a Certificate of Product Registration (CPR)?
- What are the Requirements in Securing Certificate of Product Registration?
- What are the Steps in Securing Certificate of Product Registration?
- Common Deficiencies and Reason for Application Denial
- Frequently Asked Questions
What is a Certificate of Product Registration (CPR)?
A Certificate of Product Registration (CPR) or Marketing Authorization serves as official proof of the approved application for the registration of a pharmaceutical or drug product. It serves as initial proof of the registrant’s authority to market the specific health product in accordance with the activities permitted under the License to Operate (LTO).
This requirement applies to both Over-the-Counter (OTC) and prescription medicines, which all must undergo FDA evaluation and approval prior to manufacture, importation, or distribution in the Philippine market.
Over-the-Counter (OTC) Drugs are medications that can be purchased without a prescription and are generally considered to have lower risk when used as directed. However, even if these products are easily accessible, these products still need to be registered with the FDA. This ensures that even non-prescription medicines comply with safety and quality standards before reaching consumers.
Prescription Drugs unlike over-the-counter medicines, usually contain active ingredients that have a higher chance of causing side effects or interacting with other medications. These drugs must go through a more strict and detailed evaluation process to obtain Certificate of Product Registration (CPR). The registration process involves a detailed review of clinical data, manufacturing practices, labeling, and product quality to confirm their safety and therapeutic efficacy.
What are the Requirements in Securing Certificate of Product Registration?
- Valid License to Operate
- Certificate of Analysis
- Labeling Materials (facsimile labels) that aligns with FDA’s guidelines
- Other documents (technical requirements); Certificate of Analysis, Stability Data
- Technical Dossier (ACTD Format) for Prescription Drugs
What are the Steps in Securing Certificate of Product Registration?
- Download. The Integrated Application Form (IAF) can be downloaded from the FDA website at www.fda.gov.ph.
- Fill up Form. The application form must be filled out accurately. It consists of six parts:
- General Information;
- Establishment Information;
- Product Information;
- Supporting Information;
- Sources and Clients;
- Applicant Information
If the part is appropriately filled up, a green ‘PROCEED’ will be indicated. Required fields will appear sequentially and the composed body text (in the green box) will appear.
- Email. Send an email to fdac@fda.gov.ph. In the XLS application form, the worksheet ‘Email’ composes the subject and body of the email that should be sent to fdac@fda.gov.ph. Copy and paste the appropriate fields onto the email. The XLS or XLSX file should not be attached but it will be required during submission.
- Scheduling. Within two working days, a Document Tracking Log (DTL) is sent with a schedule for submission. The FDA will determine the schedule of applications according to the priority of the Centers. Multiple applications sent in a single email may be scheduled over separate days. Requests for specific schedules will not be accommodated.
- Check. Check if all requirements are in order. Be sure that you have a checklist of requirements and that you have all the necessary documents.
- Submission. Application is filed in on schedule. Only applications scheduled for the day will be accommodated. Hard copies will no longer be required for submission.
- Pay. Payment must be made only after receiving an ACCEPTABLE pre-assessment result to avoid transfer of payment requests (in case the application is not- acceptable during the pre-assessment).
Common Deficiencies and Reason for Application Denial
- Incomplete Documentation
- Insufficient Clinical Data
- Non-compliant in labeling requirements
- Inconsistent information
- Submission of unclear/not readable files
- The files/link initially provided should be accessible, not altered/deleted and no additional documents shall be submitted until the application is endorsed to the CDRR for evaluation.
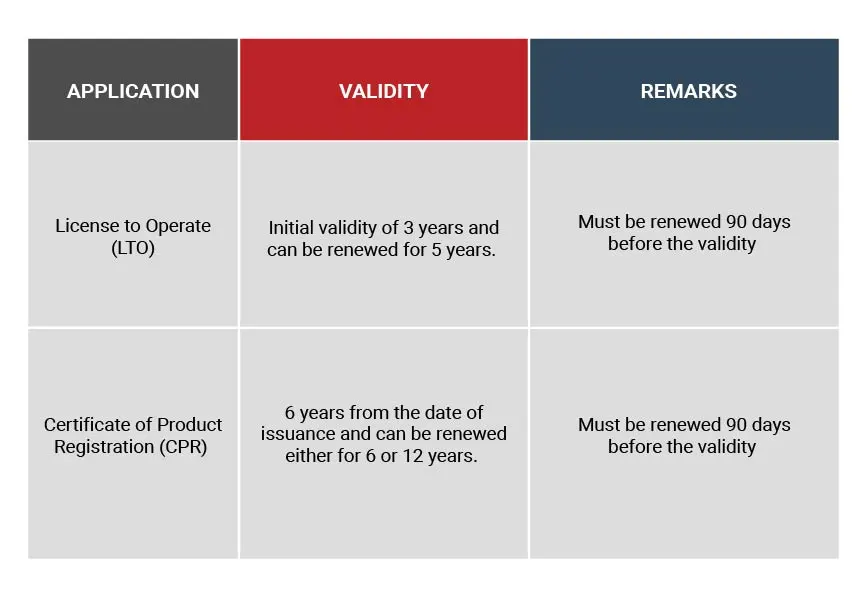
Validity and Renewal of License to Operate and Certificate of Product Registration (CPR)
Let Us Guide You Through FDA Drug Product Registration
Frequently Asked Questions
Do I need to secure a CPR/Marketing Authorization for the raw materials that will import for my own use as manufacturer?
No, raw materials used in finished drug products are not required to be registered. However, if a local drug manufacturer imports raw materials for its own use, this must be applied for as a variation specifically, an additional activity under the License to Operate (LTO) of the Drug Manufacturer or Trader.
Does the FDA allow the same brand name for the same product?
The Brand Name refers to the proprietary name given to the product by the Marketing Authorization Holder (MAH), as stated in AO No. 2016-0008. The use of the same brand name is permitted only for products that share the same generic name and are registered under the same MAH.
Are food supplements the same as drug products?
No, there are differences based on the Recommended Energy and Nutrient Intake (RENI). To be classified as food supplements, the levels of vitamins and minerals are calculated based on the percentage of RENI, in accordance with Office Order No. 22 s.1991. The maximum allowable limits are 150% RENI for water-soluble vitamins and 105% RENI for fat-soluble vitamins. For minerals, the Philippine Dietary Reference Intakes (PDRI) 2015 and ASEAN guidelines may be used as references for determining the maximum limits.
What are the officially recognized reference monograph of the FDA Philippines?
- Philippine Pharmacopoeia (PP)
- United States Pharmacopoeia (USP)
- British Pharmacopoeia (BP)
- European Pharmacopoeia (Ph. Eur.)
- Japanese Pharmacopoeia (JP)
- International Pharmacopoeia (Ph. Int.)
What is a principal certificate of product registration (PCPR)?
A Principal Certificate of Product Registration (PCPR) refers to the original or existing valid CPR issued for a specific pharmaceutical product with a particular formulation. It is granted to the owner or holder who authorizes or licenses third parties to export, import, distribute, market, and/or sell the same pharmaceutical formulation.
What is a certificate of listing of identical drug product (CLIDP)?
The Certificate of Listing of Identical Drug Product (CLIDP) is an official document issued by the FDA as proof that a pharmaceutical product has been formally listed as identical specifically in terms of its manufacturer and pharmaceutical formulation to another product already registered under a Principal Certificate of Product Registration (CPR).
Are we required to submit hard copies of original certifications during filing of application for initial registration?
Submission of hard copies of original documents is not required. However, but must be made available for inspection by the FDA officers during onsite audits.
How do you know if a procedure is FDA Philippines Approved?
Approved pharmaceutical or drug products can be verified through the FDA’s website at https://verification.fda.gov.ph.
Does the FDA allow the same brand name for the same product?
Brand Name is the propriety name given to a product by the Marketing Authorization Holder (MAH) (AO No. 2016-0008). The use of the same brand name is permitted only for products that have the same generic name and are registered under the same MAH.



Excellent Ms Domingo,
Very helpful!
Dan Agustin
Member, Research Ethics Board
East Avenue Medical Center