
Guide For Food Supplement Registration in the Philippines
The food supplement industry has seen a significant growth in the market due to more awareness on health and discovery of trend of natural ingredients and botanical plants that can be used for supplement of health. With the discovery of herbal formulation many companies, international or local, have an interest in giving the public a wide range of choices for supplements.
Many companies are entering the Philippine market for distributing the innovative products. Before they can proceed with selling the products in the market, it should have FDA registration and certificates for the public to monitor their health.
- What is Food or Dietary Supplement?
- What Qualifies as a Food or Dietary Supplement in the Philippines?
- What are the Steps for Food Supplement Registration in the Philippines?
- Important Reminders for Food Supplement Claims
- Validity and Renewal of License to Operate and Certificate of Product Registration
- Frequently Asked Questions
What is Food or Dietary Supplement?
As per FDA Philippines definition, “a Food/Dietary Supplements refers to processed food product intended to supplement the diet that bears or contains one or more of the following dietary ingredients: vitamin, mineral, amino acid, herb, or other dietary substance of botanical, animal, artificial or natural origin to increase the total daily intake in amounts conforming to the latest Philippine recommended energy and nutrient intakes or internationally agreed minimum daily requirements. It is usually in the form of capsules, tablets, liquids, gels, powders or pills and is not represented for use as a conventional food or as the sole item of a meal or diet or a replacement for drugs and medicines.”
What Qualifies as a Food or Dietary Supplement in the Philippines?
- No Therapeutic Claims – The product should not have claims to cure, prevent, mitigate, or treat any diseases. The are requiring the manufacturer to input “No Approved Therapeutic Claims”
- Recommended Dietary Intake – The content of the supplement should not exceed the maximum limit and should not imply the product is an alternative to any meal or diet.
- Dosage Form – The supplements are usually in the form of capsules, tablet, liquid form or powder and must not be in parenteral, sublingual, or other routes of administration that are not intended for direct oral intake.
- Ingredients – The supplement shall not contain any active pharmaceutical ingredients other than vitamins and minerals. The supplement usually contains botanical contents, vitamins, minerals, probiotics, and enzymes
What are the Steps for Food Supplement Registration in the Philippines?
- Secure License to Operate
International or Local companies who plan to manufacture, distribute, wholesale and trade Food products are required to obtain License to Operate to ensure that the establishment is compliant with the FDA regarding safety handling of food products. FDA may conduct pre-inspection of the facility that will handle the product.
- Identify the Product Risk Classification
It is important to note the classification of the product that you plan to register to see whether it will fall under low risk, medium risk, and high-risk classification.
- Secure Certificate of Product Registration
Once the LTO has been successfully secured, the application of the CPR may proceed following the requirements:
-
- License to Operate
- Certificate of Analysis
- Product Label and Artwork
- Stability Test
- Proforma Invoice or Distributorship Agreement
- Product Formulation
- Post-Approval
Prepare all the Certificates and requirements for inspection by the FDA. Ensure that the establishment is complying with Good Distribution Practice and ensure that all marketing and advertisement follow FDA regulations such as not making false advertisements and claims for the Food Supplement.
Important Reminders for Food Supplement Claims
When promoting a food supplement, it is important to note that claims should comply with the standards set by the Food and Drug Administration. Such claims should comply with the following:
- Claims should align with definition of the Food Supplements –
- Claims should not have any clinical and therapeutic claims – Food supplements must not be marketed to cure, prevent and mitigate disease.
- Claims should not be false and misleading – Food Supplement claims shall not be deceptive to consumers to avoid a false impression of the product benefits.
- Claims shall not be a replacement for the regular diet or meals – Claims must not suggest that the food supplement is replaced with a diet or serve as proper nutrition.
- Claims should meet the dosing recommendations stated in the evidence or references for the claimed intended effects, unless otherwise justified
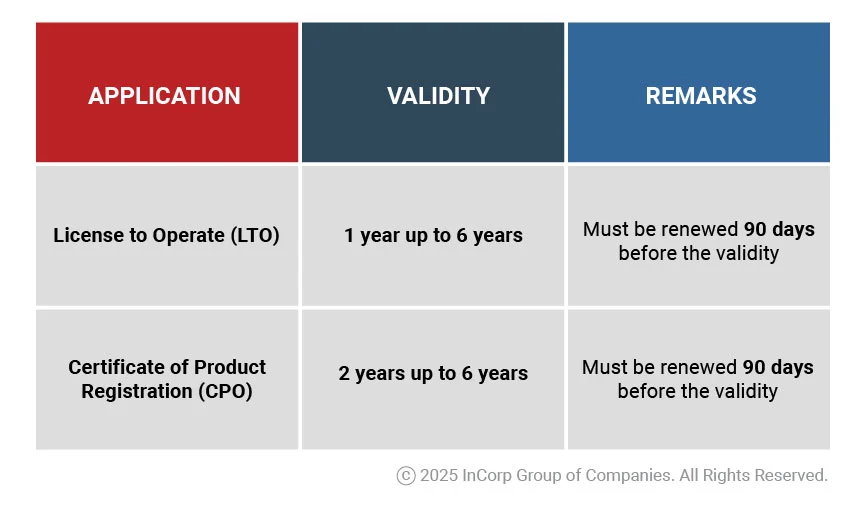
Validity and Renewal of License to Operate and Certificate of Product Registration
Let us guide you through FDA food supplement registration
Frequently Asked Questions
Which center of FDA does Food Supplements fall under?
The Center for Food Regulation and Research (CFRR) under the Food and Drug Administration is the responsible authority.
Is product registration required before selling Food Supplements in the Philippines?
Yes, Food Supplements products are required to be registered and shall have a valid Certificate of Product Registration (CPR) before they can be legally sold, imported, exported, distributed in the Philippines.
Can I import HUHS products without FDA registration?
No. Importation of HUHS products for commercial sale in the Philippines requires that the importer holds a valid LTO and CPR.
Can I use the same brand name already registered for a drug product?
No. Food supplements cannot use identical brand names to registered pharmaceutical products.
Can I advertise food supplements as a cure or treatment?
No. Food supplements cannot use identical brand names to registered pharmaceutical products.
What language should be used on the label?
Labels must be in English and/or Filipino. If a foreign language is used, an English translation must be provided.
What are the required label statements for food supplements?
All labels must include: (1) “Food/Dietary Supplement” (2) “NO APPROVED THERAPEUTIC CLAIMS”
What disclaimer is required in advertising or promotional materials?
All materials must include this standard phrase (in Filipino):
“MAHALAGANG PAALALA: ANG (NAME OF PRODUCT) AY HINDI GAMOT AT HINDI DAPAT GAMITING PANGGAMOT SA ANUMANG URI NG SAKIT.”


