
A Guide for Medical Device Registration in the Philippines
From basic instruments like thermometers and syringes to advanced equipment such as CT scanners and dialysis machines, these devices help improve the diagnosis, treatment, and monitoring of patients. In recent years, the demand for medical devices has increased due to the country’s growing population, expanding healthcare system, and rising awareness of health and wellness.
To ensure the safety, effectiveness, and proper product registration of these devices, the Philippine government, through the Food and Drug Administration, regulates their importation, distribution, and use.
- What is a Medical Device?
- What Government Agency Regulates Medical Devices in the Philippines?
- What are the Key Differences Between CMDN and CMDR?
- What are the Requirements for Registration of Medical Devices?
- Labelling Requirements for Medical Device
- Frequently Asked Questions
What is a Medical Device?
As per the FDA, Medical Device refers to any instrument, apparatus, implement, machine, appliance, implant, in-vitro reagent or calibrator, software, material, or other similar or related article intended by the manufacturer to be used alone, or in combination, for human beings for one or more of the specific purpose(s) of:
- diagnosis, prevention, monitoring, treatment or alleviation of disease;
- diagnosis, monitoring, treatment, alleviation of, or compensation for an injury;
- investigation, replacement, modification, or support of the anatomy or of a physiological process;
- supporting or sustaining life;
- preventing infection;
- control of conception;
- disinfection of medical devices;
- and providing information for medical or diagnostic purposes by means of in-vitro examination of specimens derived from the human body.
This device does not achieve its primary intended action in or on the human body by pharmacological, immunological or metabolic means but which may be assisted in intended function by such means.
What Government Agency Regulates Medical Devices in the Philippines?
In the Philippines, all medical devices must be registered with the Food and Drug Administration before they can be sold, imported, or distributed. This process ensures that only safe, effective, and quality devices reach the public, protecting health and safety across the country.
The FDA, through its Center for Device Regulation, Radiation Health, and Research (CDRRHR), oversees the evaluation and approval of medical devices. FDA classifies medical devices into four risk-based categories, following the ASEAN Medical Device Directive; they are categorized into Class A, B, C, and D.
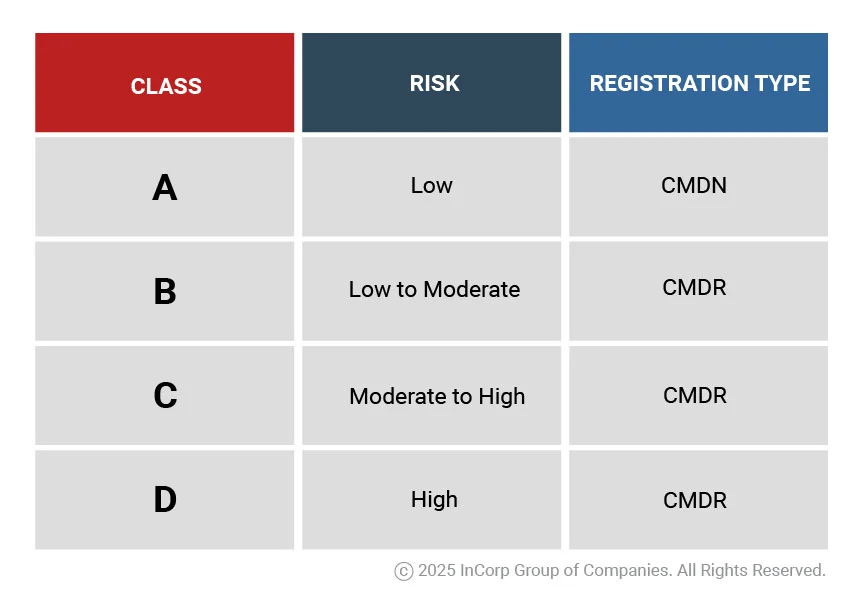
What are the Key Differences Between CMDN and CMDR?
- CMDN or Certificate of Medical Device Notification issued for Class A devices (low risk) and requires basic product information and minimal technical dossier review. Usually has faster application process time.
- CMDR or Certificate of Medical Device Registration required for Class B, C and D medical devices (moderate to high risk) and has a more comprehensive technical review and evaluation ensuring compliance with ASEAN CSDT due to higher risk
What are the Requirements for Registration of Medical Devices?
- Notarized Application Form
- Valid License to Operate
- Copy of Letter of Authorization. For imported medical devices, the Letter of Authorization must be accompanied by the original notarized declaration from the legal manufacturer or product owner, confirming that the authorization is true and correct.
- A government-issued certificate verifying the Manufacturer’s status in terms of personnel competence and facility reliability, a Quality Systems Certificate of Approval, or an ISO 13485 compliance certificate.
- For imported medical devices, the certificate must be accompanied by the original notarized declaration from the legal manufacturer or product owner confirming that the certificate is true and correct. This applies to the Certificate of Product Notification, Certificate of Product Registration, or any equivalent document issued by the regulatory agency or an accredited notified body in the country of origin attesting to the device’s safety and effectiveness.
- Colored picture of the device from all sides.
- Technical Documents in compliance with ASEAN CSDT format
Labelling Requirements for Medical Device
Product labeling typically contains the following components:
- Labels must be in English or Filipino
- Product name, brand name or trade name.
- Product code/reference number.
- Net content or pack size.
- Registration number (CMDN or CMDR).
- Manufacturer and Marketing Authorization Holder (Importer/Distributor) name and address.
- Batch/Lot code or serial number.
- Manufacture date (year/month) and Expiration date.
- Sterility status and method of sterilization, if applicable.
- Warnings, precautions, contraindications, including residual risks identified via risk analysis.
- Special handling or storage conditions.
- Intended use, especially for diagnostics or radiation-emitting devices (e.g. nature, intensity).
- Instructions for Use, if needed due to complexity.
Let Us Help You Register Your Medical Device Products With the FDA
Frequently Asked Questions
What Center of the FDA Does Medical Device Registration Fall Under?
Center for Device Regulation, Radiation Health, and Research (CDRRHR), oversees the evaluation and approval of medical devices.
What Happens if an Application for a Medical Device has Deficiencies?
The applicant is given a one-time compliance period of 90 calendar days to address the deficiencies. Non-compliance will lead to disapproval; however, a re-application may be filed within 60 calendar days following the disapproval.
What are the Grounds for Disapproval?
- The device fails to comply with established safety and effectiveness standards.
- Misrepresentation or concealment of critical data or product information.
- Any part of the device label has been altered, destroyed or removed.
- The device has biological, chemical, or physical characteristics that pose unacceptable health risks.
- Falsified documents.
- An issued CMDN or CMDR has been altered or falsified.
- All documents must be submitted in English. Any document in a foreign language that is not accompanied by a notarized English translation for legal documents and an English translation for technical documents will be disapproved.
What is the Timeline for the Registration?
As per the FDA’s Citizen’s Charter:
- Class A – 25 working days*
- Class B – 80 working days*
- Class C and D – 110 working days*
*Provided that the application is complete and correct with no Notice of Deficiencies issued.
How Long is a CMDN or CMDR Valid?
Both are valid for 6 years from the date of issuance and can be renewed either for 6 or 12 years.
Can CMDN Be Upgraded to CMDR?
No, it cannot be upgraded unless the FDA has reclassified or issued a latest list of registrable devices.
What Documents are Checked During FDA Inspections?
All licensed retailers shall be subjected to routine or spot check inspection during operating hours by authorized FDA personnel. The following documents shall be verified during inspection:
- Valid LTO
- Copies of CMDN/CMDR (as applicable)
- Proof of business name registration
- Business Permit
- Credentials of qualified person (i.e., Valid PRC)
- Standard Operating Procedures
- Risk Management Plan
What Happens if a Device is not yet Listed as Registrable?
If a device is not yet on the FDA’s list of registrable devices, the manufacturer/importer/distributor is not yet required to obtain a Certificate of Medical Device Notification (CMDN) or Certificate of Medical Device Registration (CMDR).
If you are unsure whether a device needs a registration or which category it may fall under, you can inquire or submit a product classification inquiry to the FDA CDRRHR (cdrrhr.lrd@fda.gov.ph) or with the Food and Drug Action Center (fdac@fda.gov.ph).


